Development is Risky, Costly, & Stagnant

Stifles Innovation
in the healthcare industry
Unnecessary Risks and Losses
in research and development
Impedes
the creation for patient treatment options

Smart Science Platform
The PHDC Smart Science Platform enables real-time, on-demand test development which saves time and cost in research and development. PHDC is a fully integrated data-to-diagnostic production platform that’s taking the risk out of clinical R&D projects.
- Distribute returns to shareholders
- Validation Both analytical and clinical
- Leverage research and development from others
- IP Protection Patent trade secrets
- Verification CLIA and LDT validation
- Commercialize Create market LDT/FDA
From Preliminary Launch to FDA Approval

Smart Diabetes
Diabetes Prevent is more than a pioneering risk evaluation genetic test. It aims to help you control and prevent type 2 diabetes.

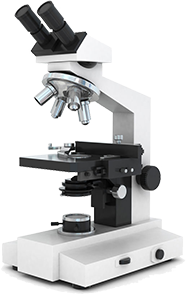
Smart Path
Rapid Slide-Free Pathology Instrument with Potential to Fundamentally Improve Diagnostic Microscopy.

Smart VascularDX
Endothelial Damage in Arteries & Guide Therapy. 2.5x More Sensitive than hsCRP for Inflammation.

Smart KidneyDX
A biomarker-based algorithm that confirms the diagnosis, grades the tumor, and predicts risk of metastases

Smart LungDX
Molecular Diagnostic with a 93% Sensitivity & 57% Specificity. PHDC will gain clinical validation for this technology with less than $2M in R&D spending, in just 12 months.

Smart CellDX
Cardiovascular Disease (CVD) tests currently lack specificity with only cholesterol and lipid panel results
What Patients Say About Us

Dr. john Martin
Cancer ResearchThere are many variations of passages of Lorem Ipsum available, but the majority have suffered alteration in some form, by injected humour, or randomised words which don't look even believable.

Dr. john Martin
Cancer ResearchThere are many variations of passages of Lorem Ipsum available, but the majority have suffered alteration in some form, by injected humour, or randomised words which don't look even believable.

Dr. john Martin
Cancer ResearchThere are many variations of passages of Lorem Ipsum available, but the majority have suffered alteration in some form, by injected humour, or randomised words which don't look even believable.

Dr. john Martin
Cancer ResearchThere are many variations of passages of Lorem Ipsum available, but the majority have suffered alteration in some form, by injected humour, or randomised words which don't look even believable.
News & Media
- admin
- news
SmartHealth Dx Featured in Cover Story of Med Tech Outlook Magazine’s Pathology Special Edition
IRVINE, Calif., June 13, 2023 /PRNewswire/ — Smart Health Diagnostics Company (“SHDx” or “the Company”), a developer of precision diagnostic tests that address unmet clinical needs in pathology and vascular inflammation for advancing today’s standards of care, announced that CEO and Chairman Mitch Levine is featured in the June 8, 2023 edition of Med Tech Outlook magazine in its special Pathology edition. The article discusses the Company’s precision diagnostics instrument designed for surgical pathology that has the potential to improve standards of practice in diagnostic microscopy fundamentally. The product, called SmartPath Dx, a desktop digital imaging system that employs proprietary technology to produce a non-destructive, slide-free evaluation of fresh or preserved tissue samples in minutes for a real-time pathology diagnosis. The Company intends to use SmartPath Dx in multiple capacities: for rapid on-site evaluation of biopsies and cancer margins; to reduce time spent preparing and evaluating samples in the frozen section room; in Mohs surgical procedures for treating skin cancer; and for veterinary medicine. As quoted in the article, Mitch Levine states, “Our technology has been termed the greatest innovation in pathology instrumentation in the last 100 years, as it is capable of speeding up the process from sample collection to diagnosis for patients undergoing biopsy or surgical procedures.” The Company believes key benefits of the SmartPath Dx instrument include: Results within minutes rather than hours or days, reducing time, labor, and equipment costs. Water-soluble, non-destructive techniques leave the tissue intact. Technicians are not exposed to toxic chemicals. The instrument can be used in various healthcare and research settings such as surgical suite, point of care diagnosis, frozen section room, pathology dept, interventional suites, women’s health, dermatology, veterinary, and research & development. The software is optimized for global digital image distribution. About SmartHealth Dx SmartHealth Dx (“SHDx”) is a specialty diagnostics development platform company that develops, manufactures, and distributes unique medical tests combining science, technology, and proprietary analytics which aim to detect high-risk diseases with significant unmet medical needs. SHDx’s laboratory information systems use data from multiple sources, including proteomics, genetics, metabolic, biochemistry, phenotype, and imaging, to address the most challenging clinical problems. Morningstar Laboratories, a SmartHealth Dx company, is a CLIA-Certified and CAP Accredited laboratory offering comprehensive and customized services based on Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) regulations and ISO 15189 standards. To learn more, visit SHDx at smarthealthdx.com, Twitter, Facebook, and LinkedIn. Forward-Looking Statements Forward-looking statements in this press release are based on our future expectations, plans prospects, and assumptions regarding matters that are not historical facts, may constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. The words “termed,” “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward-looking statements. Therefore, we caution you against relying on any of these forward-looking statements. They are neither statements of historical fact nor guarantees or assurances of future performance. Any forward-looking statement made by us in this document speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments, or otherwise, except as may be required by law. SOURCE SmartHealth Diagnostics, Inc.
- admin
- news
Specialty Diagnostics Technology and Solutions Provider Unveils New Brand Identity as SmartHealth Diagnostics
SmartHealth name unifies multi-function operating platform with smart science diagnostics product suite under common brand
- admin
- news
SmartHealth Diagnostics Appoints Accomplished Corporate and Finance Leader Mitch Levine as President, Chief Financial Officer, and Director
Highly regarded diagnostics industry executive and capital markets expert to guide planned growth and value creation for evolutionary data-to-diagnostics platform company
- admin
- news
Diagnostic Tests to Improve Patient Care
Company announces its business strategy including test development, commercialization, intellectual property, and reimbursement initiatives
- admin
- news
Predictive Health Diagnostics Company Welcomes Susie Lu as President of Laboratory Services for All Company Businesses
Susie Lu as President of Laboratory Services for all Predictive Health Diagnostics Company businesses.
- admin
- news
Predictive Health Diagnostics Company Appoints 30-Year Diagnostic Imaging Executive Larry Dentice as Chief Commercial Officer and Director
Larry Dentice as Chief Commercial Officer. Mr. Dentice has also stepped into a board role following the departure of longtime board member Munira Mubarak.
- admin
- news
Predictive Health Diagnostics Company’s Morningstar Laboratories Receives Clinical Laboratory Permit from Pennsylvania Department of Health
Morningstar to bring much needed laboratory service resources, including COVID-19 antibody testing, to urban and rural communities statewide
- admin
- news
Predictive Health Diagnostics Company’s PULS Cardiac Test Plays Key Role in Detecting Vascular Inflammation Common After Receiving COVID-19 Vaccines
PULS Test vascular inflammation biomarkers and scores reveal risk of developing acute coronary syndrome (ACS) which can be increased in mRNA COVID-19 vaccinated patients
- admin
- news
Predictive Health Diagnostics Company Expands Smart Science Diagnostics Platform with Acquisition of MUSE Microscopy Inc.
AI-derived imaging platform expands Company’s test development expertise to create next generation diagnostics for pathology
- admin
- news
Predictive Health Diagnostics Company Announces Rollout of Proprietary LIS Cloud-Based Laboratory Information System Software
LIS system enables scalable functionality and big data analysis capabilities
- admin
- news
Predictive Health Diagnostics Company’s Morningstar Laboratories Reports Expanding In-Network Provider Participation, Achieves Accreditation from College of American Pathologists (CAP)
Expanding in-network status reflects strong demand and utilization of the PULS Cardiac Testâ„¢ and DIABETESpredictâ„¢ in healthcare settings nationwide
- admin
- news
Predictive Health Diagnostics Company Announces Partnership with Madison Core Laboratories for PULS Cardiac Testâ„¢ Distribution to 30+ Healthcare Facilities
Distribution targeted to high at-risk co-morbid patient populations